Extracellular vesicles (EVs)
Extracellular vesicles (EVs) are nanoparticles surrounded by lipid bylayers that are released from eucaryotic and procaryotic cells. Their size ranges from 30nm to 4µm. The first evidence of these subcellular structures was found in 1956 by Don Fawcett during electron microscopic examination of tumor cells. Due to this fact and the lack of methods to analyse particles in this subcellular size range it took about five decades before their role was further investigated. Since the early 2000s, the number of publications on EVs has been increasing drastically.
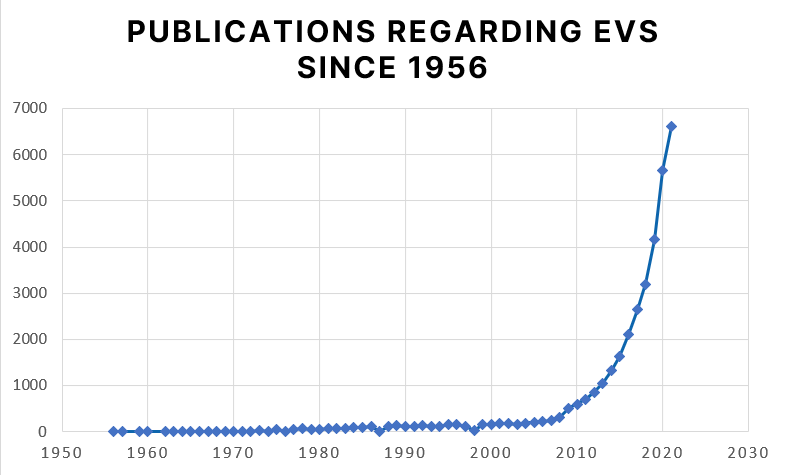
Source: https://pubmed.ncbi.nlm.nih.gov/?term=extracellular+vesicle&timeline=expanded
Nanoparticle Tracking Analysis (NTA) thus contributed significantly to the understanding of the biological relevance of extracellular vesicles, as it was the first method making EVs visible in their natural dispersed state.
Using Nanoparticle Tracking Analysis and other techniques, multiple publications have shown that extracellular vesicles are involved in the regulation of cellular activities. They fulfill their functions by transporting DNA, RNA, proteins, lipids and metabolites from their origin to their target cells.
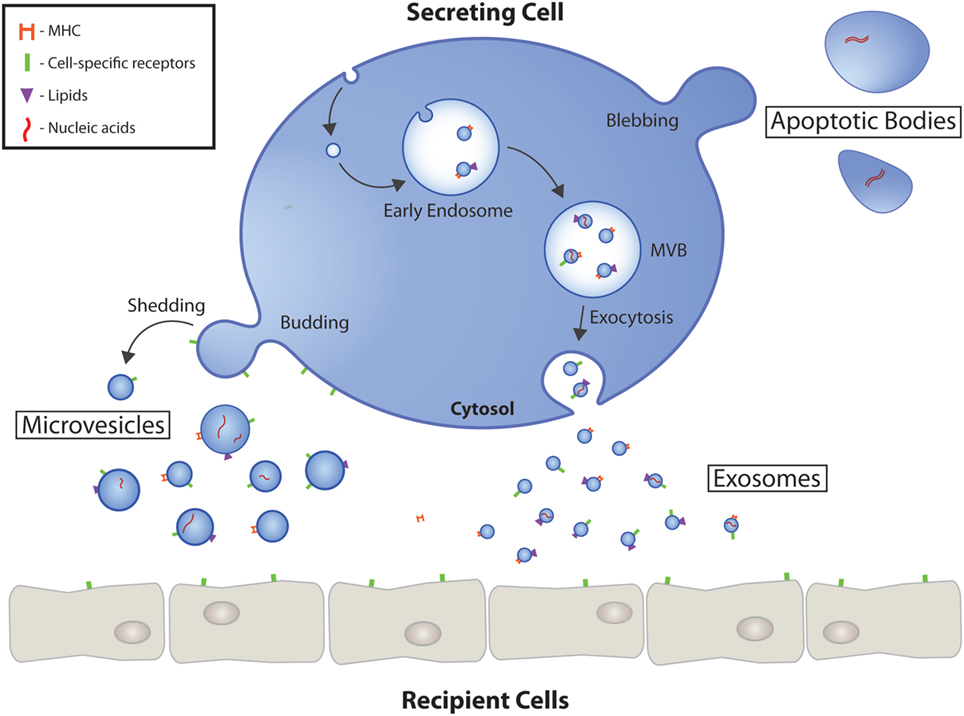
EVs are divided into exosomes, microvesicles and apoptotic bodies depending on their origin and genesis. Exosomes are the smallest EVs with a size of 30 – 130 nm. They are of endosomal origin and must undergo exocytosis to be released from their cell of origin. In addition, there are microvesicles that are up to 1000 nm in size and are formed by plasma membrane excrescence. The largest EVs are the apoptotic bodies, which are formed during programmed cell death.
Using Nanoparticle Tracking Analysis and other techniques, multiple publications have shown that extracellular vesicles are involved in the regulation of cellular activities. They fulfill their functions by transporting DNA, RNA, proteins, lipids and metabolites from their origin to their target cells.
EVs are divided into exosomes, microvesicles and apoptotic bodies depending on their origin and genesis. Exosomes are the smallest EVs with a size of 30 – 130 nm. They are of endosomal origin and must undergo exocytosis to be released from their cell of origin. In addition, there are microvesicles that are up to 1000 nm in size and are formed by plasma membrane excrescence. The largest EVs are the apoptotic bodies, which are formed during programmed cell death.
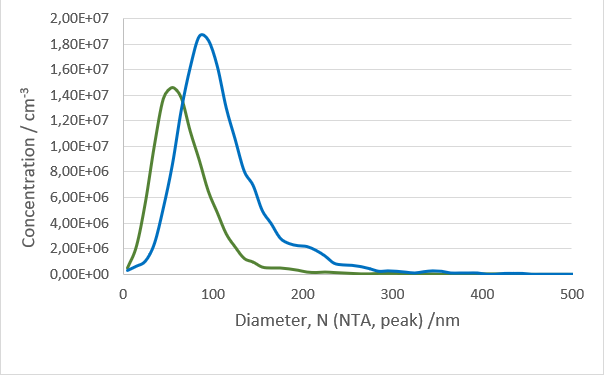
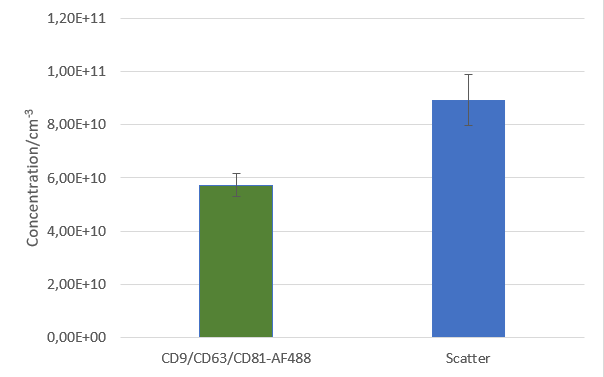
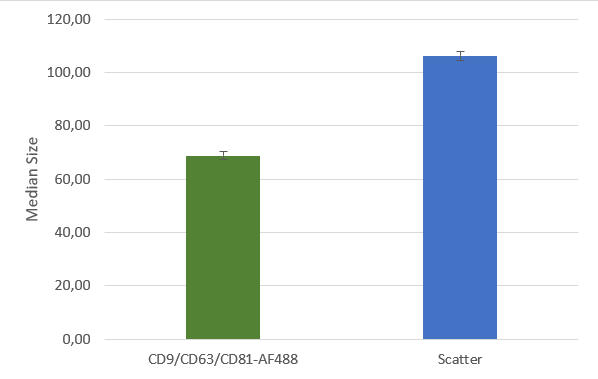
After isolation, the obtained vesicles should be checked with NTA, since this is the easiest and fastest technique providing reliable information about the size and concentration of the isolated vesicles. Additionally, purity should be checked by fluorescence Nanoparticle Tracking Analysis (F-NTA) using specific dyes to label the vesicles of interest. For exosome isolations, triple staining with antibodies directed against CD9, CD63 and CD81 is recommended providing detailed information on the size, concentration and percentage of the exosome fraction. To unravel the origin of the obtained exosomes, counterstaining with a specific cell marker can be performed for analysis in the ZetaView® TWIN or QUATT multilaser systems.
Afterwards, different methods are used to determine the contribution of the EVs to the disease. For example, their cargo is analysed to understand in which way they influence the behaviour of their target cells. Here, the proteins and nucleic acids are of greatest interest since they have the ability to influence gene expression. Therefore, the vesicles cargo is isolated using solubilizing agents to destroy the surface of the extracellular vesicles. Subsequently, different proteomic approaches can be performed. Protein identification by comparing the isolated proteins with a protein library is often used as a starting point. By comparing samples from healthy and diseased patients, candidates of interest can be identified.
The quantity of these candidates can then be analysed by methods like Western blotting, ELISA, flow cytometry or F-NTA. Of greatest interest is, of course, the functional proteomics analysis, which identifies the biological function of the identified proteins. The method spectra here is extremely broad, since protein functions can differ enormously. As a first indication, the interaction of proteins with other well-characterized proteins can be analysed using methods like co-immunoprecipitation or fluorescence-based microscopy techniques such as FLIM or FRET.
However, even if the biological function cannot be unravelled, the identified proteins can be used as biomarker for a specific disease. The protein content of the EVs can be analysed to determine a patient’s disease state or monitor a patient’s response to a treatment. Therefore, the isolated EVs can be stained using specific antibodies and then analysed using F-NTA or other methods. Because the isolation of EVs is not invasive it has multiple obvious advantages over biopsy.
EXTRACELLULAR VESICLES AS A THERAPEUTIC APPROACH
Since it is known that EVs can effectively target their recipient cell and release cargo to alter cellular functions, they are an obvious and effective vehicle for therapeutics. Because of their direct targeting to the recipient cells, the administered drug concentration can be decreased, resulting in reduced toxicity, fewer side effects and lower treatment costs. Of note is the ability of the EVs to cross natural barriers such as tissue, cellular and intracellular barriers. Even the most challenging barrier for therapeutic approaches, the blood brain barrier, can be overcome by EVs.
There are two generally different ways in which EVs can be used for therapeutic approaches. Either their endogenous potential is used, or they serve as vehicles for the transport of artificial cargo.
The Endogenous Potential of Extracellular Vesicles
Their endogenous potential can be used, for example, as cancer vaccine. Tumor EVs extracted from cell lines can thus elicit anti-tumor immune response, which has already been successfully tested in clinical trials.
Additionally, EVs extracted from specific tissues can be used to induce or support tissue regeneration after injury or surgery.
Extracellular Vesicles as Vehicles for the Transport of Artificial Cargo
For the use of EVs as vehicle for drugs, an effective way of loading must be used. In general, there are two ways: either the cargo is loaded during the biogenesis of the EVs by the parental cell or exogenously after the EV isolation.
Especially for chemotherapy, EV mediated direct targeting of tumor cells would be of great advantage, as it would solve many problems such as systemic effects due to its high cytotoxicity and rapid clearance. Synthetic vesicles have been developed for that purpose some of which are already clinically approved.
The production of artificial vesicles, in contrast to the generation of EVs, can be controlled and standardized much easier, what guarantees consistent product quality. However, in terms of efficacy, natural EVs are superior, as they are generally less cytotoxic and much more effective in targeting.
For product safety the production of vesicles must monitored. The Nanoparticle Tracking Analysis technique is here of great help since the concentration and purity can be analysed within minutes. Additionally, the loading can be analysed by the use of F-NTA.
การวิเคราะห์ shelf life และความคงตัวของผลิตภัณฑ์ ด้วยเทคนิค SMLS ทำให้ได้ผลการวิเคราะห์อย่างรวดเร็ว และมีความแม่นยำมากกว่าวิธีการตรวจวัดด้วยสายตา
DSC รุ่นใหม่ล่าสุดจาก Hitachi High-Tech ที่จะทำให้งานของคุณง่ายขึ้น และถูกต้องมากขึ้น
Lorem ipsum dolor sit amet, pri et feugiat consulatu. Eu per ceteros platonem. Ea dictas legendos ius.
Lorem ipsum dolor sit amet, pri et feugiat consulatu. Eu per ceteros platonem. Ea dictas legendos ius.
Lorem ipsum dolor sit amet, pri et feugiat consulatu. Eu per ceteros platonem. Ea dictas legendos ius.










